We are developing soft neural interface platforms that can control neural interfaces and integrate data transmission, signal processing, and power management. These works involve fabrication of flexible electronic systems and development of novel antenna systems and integrated circuit systems. In parallel, we are studying novel methods to maximize wireless power transmission into biological tissues. The followings are ongoing projects
-
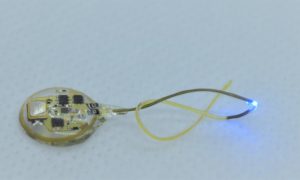
Wireless gastric optogenetic gastric implant for the study of PNS
Images of organ specific, multimodal optoelectronic device (Left) and the stomach with the device implanted(Right)
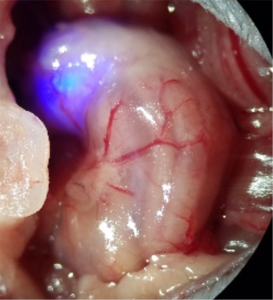
Optogenetic control of Calca neurons inhibits appetite
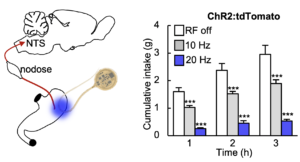
In vivo demonstration of optogenetic control of appetite
-
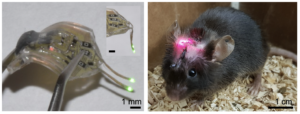
Soft, wireless optogenetic brain implants for multiplexing brain dynamics
Images of wireless dual-channel brain implant (left) and animal with a device implanted (Middle).
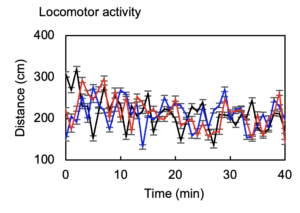
In vivo demonstration of actuating mechanisms by which channels are selected.
Simultaneous/independent control of multiple animals
-
Low power battery-free wireless telemetry for high-throughput phenotyping of neural pathways
-
Closed loop controlled wireless multichannel photometry system
Fear behavior with wireless optoinhibition of the amygdala
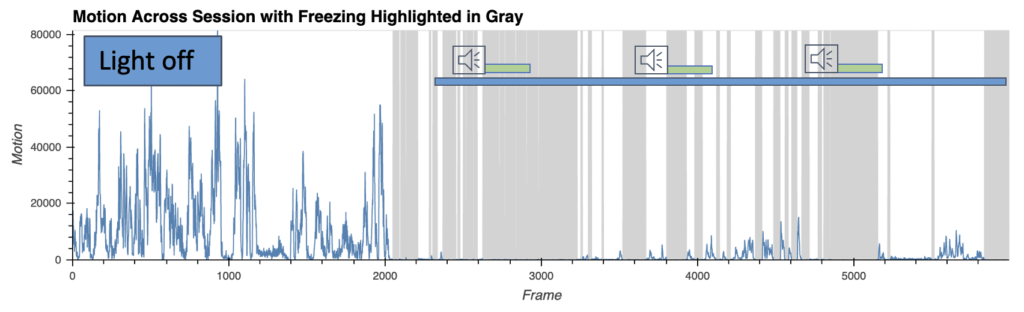
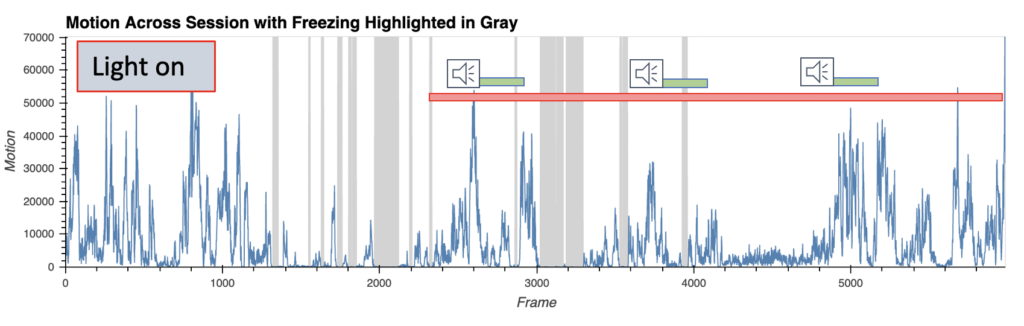
- AI-enabled wireless telemetry for the photodynamic therapy